 The statistical principles of equivalence trials and non-inferiority, as well as the special case bioequivalence, were presented. From a statistical perspective, being non-inferior or bioequivalent means to be only irrelevantly inferior or different, respectively. Based on this idea, the null hypotheses for non-inferiority or bioequivalence are shown.
The statistical principles of equivalence trials and non-inferiority, as well as the special case bioequivalence, were presented. From a statistical perspective, being non-inferior or bioequivalent means to be only irrelevantly inferior or different, respectively. Based on this idea, the null hypotheses for non-inferiority or bioequivalence are shown.
The test decision, based on confidence intervals and the duality to the test problem, were discussed. A simple example of how to determine the acceptance range in a clinical trial was introduced. Problems with the constancy assumption and advances in standard of care were shown based on examples.
Further thoughts on populations, sample size and regulatory aspects as well as practical implementation in the protocol or statistical analysis plan were depicted.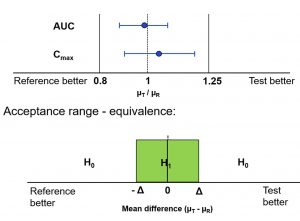

Recent Comments