 In the past few years, more and more articles dealing with Bayesian statistics and Bayesian designs in clinical trials were published. Such designs are being developed especially for clinical trials with small number of patients, where it is crucial to get the most information from the limited available data.
In the past few years, more and more articles dealing with Bayesian statistics and Bayesian designs in clinical trials were published. Such designs are being developed especially for clinical trials with small number of patients, where it is crucial to get the most information from the limited available data.
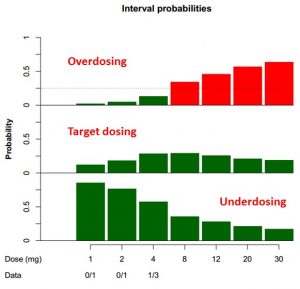
The Bayesian Logistics Regression Model (BLRM), presented here in the context of oncology dose escalation trials, fulfills these requirements. This design allows to estimate the probabilities that pre-defined treatment doses are underdosing, target dosing, or overdosing, according to criteria such as the number of Dose Limiting Toxicities (DLT) observed. Using prior information (literature, earlier trials) as well as current data, in a logistic regression model, this design recommends the next dose to be tested until the Maximum Tolerated Dose (MTD) is achieved.
The talk includes a presentation of the model, guided with an example, as well as possible tools to evaluate the model. Several possible extensions of this model are briefly presented.
Bayesian designs in phase II trials are mentioned at the end of the presentation, in order to show the constant development taking place in this domain.

Recent Comments